Sucralose = Splenda
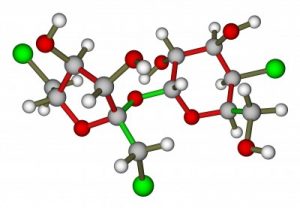
Sucralose (marketed as Splenda) became a “low-calorie” sugar for you and your children to eat and drink after undergoing a complicated process in the “sugar lab.” Here’s Splenda’s chemical formula: 1,6-dichloro-1, 6-dideoxy-BETA-D-fructofuranosyl-4-chloro-4-deoxy-alpha-D-galactopyranoside. Basically, sucralose (Splenda) has been marketed as just benign sugar with a touch of chlorine, but you might disagree when you read the other side of this equation.
Using a complex process involving dozens of chemicals that you and I can barely pronounce – let alone consume – I have listed the actual process for producing this diet sweetener. I highlighted the “chemicals used” in bold.
According to the Splenda International Patent A23L001-236 and PEP Review #90-1-4 (July 1991), sucralose is synthesized by this five-step process:
1. sucrose is tritylated with trityl chloride in the presence of dimethylformamide and 4–methylmorpholine and the tritylated sucrose is then acetylated with acetic anhydride,
2. the resulting TRISPA (6,1′,6′-tri-O-trityl-penta-O-acetylsucrose) is chlorinated with hydrogen chloride in the presence of toluene,
3. the resulting 4-PAS (sucrose 2,3,4,3′,4′-pentaacetate) is heated in the presence of methyl isobutyl ketone and acetic acid,
4. the resulting 6-PAS (sucrose 2,3,6,3′,4′-pentaacetate) is chlorinated with thionyl chloride in the presence of toluene and benzyltriethylammonium chloride, and
5. the resulting TOSPA (sucralose pentaacetate) is treated with methanol (wood alcohol, a poison) in the presence of sodium methoxide to produce sucralose.
The Hidden Chemicals in Splenda
The chemicals used to synthesize sucralose in the five-step process (above) are more than just simple benign sugar with a touch of chlorine. The chemicals used to make sucralose (Splenda) are:
- Acetone
- Acetic acid
- Acetyl alcohol
- Acetic anhydride
- Ammonium chloride
- Benzene
- Chlorinated sulfates
- Ethyl alcohol
- Isobutyl ketones
- Formaldehyde
- Hydrogen chloride
- Lithium chloride
- Methanol
- Sodium methoxide
- Sulfuryl chloride
- Trityl chloride
- Toluene
- Thionyl chloride
It’s time to admit that there is no free ticket to eating all the sugar-free products you desire without paying the high price of harming your body in the long run. Laboratory chemicals are not the sugar-free solution to health and wellness.
It certainly doesn’t sound very appetizing to me.